
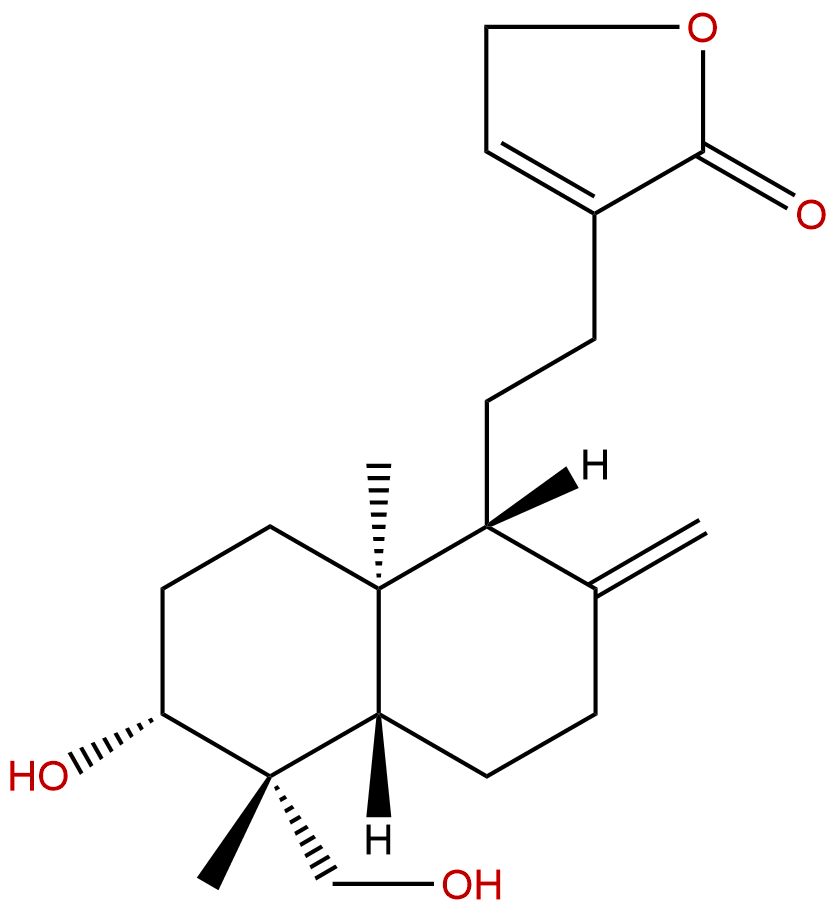
14-DeoxyandrographolideCAS No.:4176-97-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1811 |
| Formula: | C20H30O4 |
| Mol Weight: | 334.456 |
Product name: 14-Deoxyandrographolide
Synonym name:
Catalogue No.: BP1811
Cas No.: 4176-97-0
Formula: C20H30O4
Mol Weight: 334.456
Botanical Source: Andrographis paniculata
Physical Description: Cryst.
Type of Compound: Diterpenoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
The product could be supplied from milligrams to grams. Inquire for bulk scale.
We provide solution to improve the water-solubility of compounds, thereby facilitating the variety of activity tests and clinic uses.
For Reference Standard and R&D, Not for Human Use Directly.
Description:
14-Deoxyandrographolide has hepatoprotective activity, mediates activation of adenylate cyclase-cAMP signaling leading to up-regulation of cNOS, may provide a promising approach in the prevention of liver diseases during chronic alcoholism. It desensitizes hepatocytes to TNF-alpha-mediated apoptosis through the release of TNFRSF1A.
References:
Br J Pharmacol. 2010 Aug;160(7):1823-43.
14-Deoxyandrographolide desensitizes hepatocytes to tumour necrosis factor-alpha-induced apoptosis through calcium-dependent tumour necrosis factor receptor superfamily member 1A release via the NO/cGMP pathway.
Andrographis paniculata (AP) has been found to display hepatoprotective effect, although the mechanism of action of the active compounds of AP in this context still remains unclear. Here, we evaluated the hepatoprotective efficacy of 14-Deoxyandrographolide (14-DAG), a bioactive compound of AP, particularly its role in desensitization of hepatocytes to tumour necrosis factor-alpha (TNF-alpha)-induced signalling of apoptosis.
METHODS AND RESULTS:
TNF-alpha-mediated ligand receptor interaction in hepatocytes in the presence of 14-DAG was studied in vitro in primary hepatocyte cultures, with the help of co-immunoprecipitation, confocal microscopy and FACS analysis. Events associated with 14-DAG-induced TNFRSF1A release from hepatocytes were determined using immunoblotting, biochemical assay and fluorimetric studies. Pulse-chase experiments with radiolabelled TNF-alpha and detection of apoptotic nuclei by terminal transferase-mediated dUTP nick-end labelling were performed under in vivo conditions. 14-DAG down-regulated the formation of death-inducing signalling complex, resulting in desensitization of hepatocytes to TNF-alpha-induced apoptosis. Pretreatment of hepatocytes with 14-DAG accentuated microsomal Ca-ATPase activity through induction of NO/cGMP pathway. This resulted in enhanced calcium influx into microsomal lumen with the formation of TNFRSF1A-ARTS-1-NUCB2 complex in cellular vesicles. It was followed by the release of full-length 55 kDa TNFRSF1A and a reduction in the number of cell surface TNFRSF1A, which eventually caused diminution of TNF-alpha signal in hepatocytes.
CONCLUSIONS:
Taken together, the results demonstrate for the first time that 14-DAG desensitizes hepatocytes to TNF-alpha-mediated apoptosis through the release of TNFRSF1A. This can be used as a strategy against cytokine-mediated hepatocyte apoptosis in liver dysfunctions.
Food Chem Toxicol. 2013 Sep;59:236-48.
14-Deoxyandrographolide targets adenylate cyclase and prevents ethanol-induced liver injury through constitutive NOS dependent reduced redox signaling in rats.
Chronic alcoholism is one of the most common causes of liver diseases worldwide. Nitric oxide (NO) has been proposed to have potential for clinical application against chronic hepatocellular injuries. However, mechanisms underlying hepatoprotective functions of NO in ethanol-induced apoptosis are largely unknown.
METHODS AND RESULTS:
Sprauge-Dawley rats were exposed to ethanol for 8 weeks. Half of the ethanol-fed animals received 14-Deoxyandrographolide (14-DAG) treatment for the last 4 weeks of study. Preventive effect of 14-Deoxyandrographolide against ethanol-induced hepatotoxicity involved constitutive nitric oxide synthase (cNOS) activation followed by up-regulation of γ-glutamylcysteine synthetase activity and reduced oxidative stress. 14-Deoxyandrographolide acted as activator of adenylate cyclase and modulated cyclic AMP (cAMP) mediated expression of caveolin-1 and calmodulin.
CONCLUSIONS:
Our results suggest that, protective effect of 14-Deoxyandrographolide against ethanol-induced hepatic injury is based on its ability to reduce oxidative stress through cNOS dependent improvement of redox status. 14-Deoxyandrographolide mediated activation of adenylate cyclase-cAMP signaling leading to up-regulation of cNOS may provide a promising approach in the prevention of liver diseases during chronic alcoholism.