
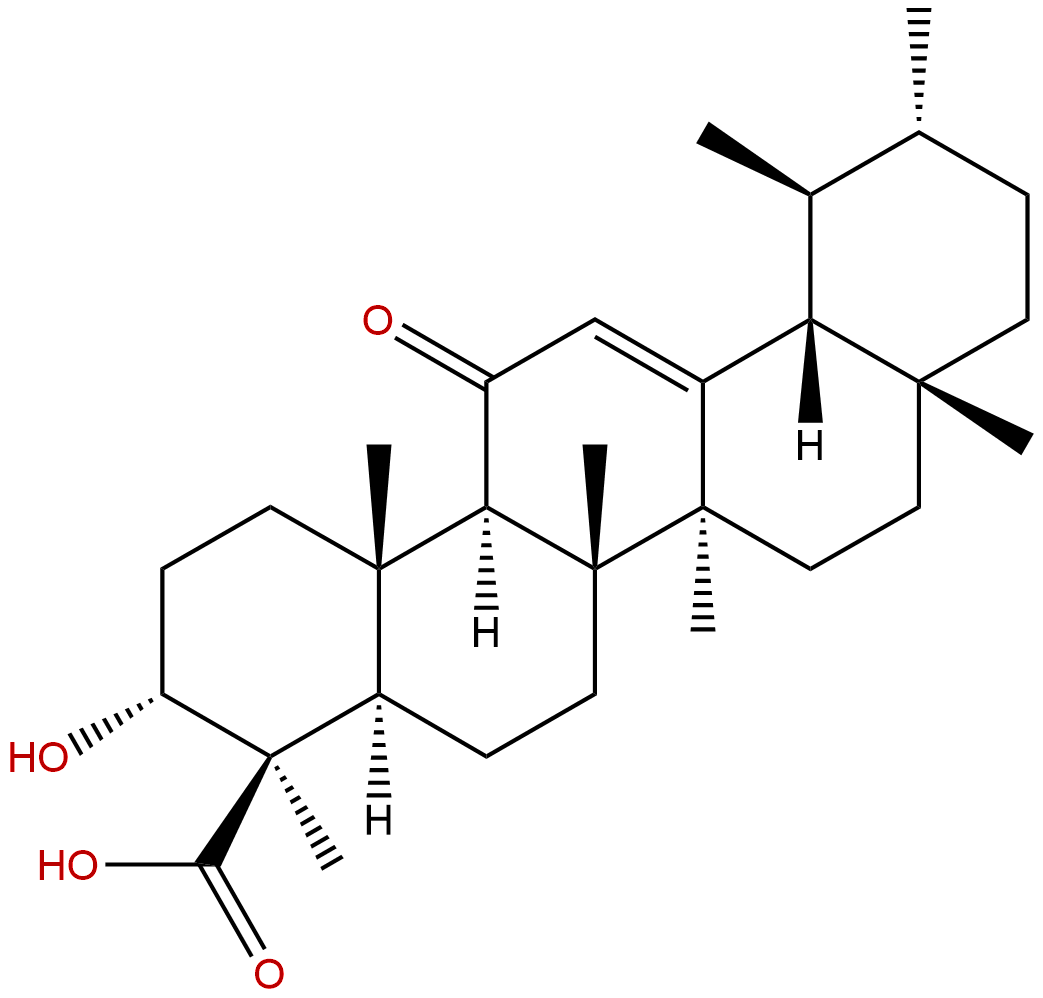
11-Keto-beta-boswellic acidCAS No.:17019-92-0
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0018 |
| Formula: | C30H46O4 |
| Mol Weight: | 470.694 |
Product name: 11-Keto-beta-boswellic acid
Synonym name: 11-Keto-β-boswellic acid; 3-Hydroxy-11-oxo-12-ursen-24-oic acid
Catalogue No.: BP0018
Cas No.: 17019-92-0
Formula: C30H46O4
Mol Weight: 470.694
Botanical Source: Boswellia carteri and Boswellia serrata (Indian olibanum)
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
11-Keto-beta-boswellic acid, a novel Nrf2 activator, and a selective 5-lipoxygenase (5-LOX) inhibitor; it exerts dose dependent cardioprotective effect manifested by dose-dependent reduction in serum lactate dehydrogenase; and it can increase the Nrf2 and HO-1 expression, which provides protection against oxygen and glucose deprivation (OGD)-induced oxidative insult. 11-Keto-beta-boswellic acid possesses significant anti-inflammatory, and anti-tumoral activities.
References:
Phytochemistry. 2013 Dec;96:330-6.
Biotransformation of 11-keto-β-boswellic acid by Cunninghamella blakesleana.
11-Keto-beta-boswellic acid (KBA), as one of the active constituents in the gum resin of Boswellia serrata, possesses significant biological activities including anti-inflammatory activity. However, its extensive metabolism and low polarity has limited the systemic availability of 11-Keto-beta-boswellic acid. The present research was aimed to obtain and explore the various possible derivatives of 11-Keto-beta-boswellic acidthrough biotransformation by Cunninghamella blakesleana AS 3.970.
METHODS AND RESULTS:
A total of ten transformed compounds were isolated and purified, and their chemical structures were characterized as 7β-hydroxy-11-keto-β-boswellic acid; 7β, 15α-dihydroxy-11-keto-β-boswellic acid ; 7β, 16β-dihydroxy-11-keto-β-boswellic acid; 7β, 16α-dihydroxy-11-keto-β-boswellic acid; 7β, 22β-dihydroxy-11-keto-β-boswellic acid; 7β, 21β-dihydroxy-11-keto-β-boswellic acid; 7β, 20β-dihydroxy-11-keto-β-boswellic acid; 7β, 30-dihydroxy-11-keto-β-boswellic acid; 3α, 7β-dihydroxy-11-oxours-12-ene-24, 30-dioic acid and 3α, 7β-dihydroxy-30-(2-hydroxypropanoyloxy)-11-oxours-12-en-24-oic acid by various spectroscopic methods. The biotransformation processes include hydroxylation, oxidation and esterification. Primary structure-activity relationships (SAR) of inhibitory effects on NO production in RAW 264.7 macrophage cells are discussed.
Eur J Med Chem. 2015 Jul 15;100:98-105.
11-Keto-boswellic acid derived amides and monodesmosidic saponins induce apoptosis in breast and cervical cancers cells.
Beta-boswellic acids are considered the main bioactive components of frankincense. Their potential to act as cytotoxic agents, as well as that of their derivatives remained unexploited so far.
METHODS AND RESULTS:
In this study we were able to prepare derivatives of 11-keto-β-boswellic acid (KBA) that showed lower IC50 values as determined by a sulphorhodamine B (SRB) assay using several different human tumour cell lines. Monodesmosidic saponins of KBA are as cytotoxic as 3-acetyl-KBA. The presence of a free hydroxyl group at position C-3 seems to lower cytotoxicity while the presence of an amide function at C-24 improves cytotoxicity. The most active compound of this series gave IC50 values as low as 4.5 μM.
CONCLUSIONS:
Cell death proceeded mainly via apoptosis.
Mol Neurobiol. 2015 Dec;52(3):1430-1439.
Posttreatment with 11-Keto-β-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism.
Oxidative stress is well known to play a pivotal role in cerebral ischemia-reperfusion injury. The nuclear factor erythroid-2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway has been considered a potential target for neuroprotection in stroke. 11-Keto-beta-boswellic acid(KBA) is a triterpenoid compound from extracts of Boswellia serrata. The aim of the present study was to determine whether KBA, a novel Nrf2 activator, can protect against cerebral ischemic injury.
METHODS AND RESULTS:
Middle cerebral artery occlusion (MCAO) was operated on male Sprague-Dawley rats. KBA (25 mg/kg) applied 1 h after reperfusion significantly reduced infarct volumes and apoptotic cells as well as increased neurologic scores at 48 h after reperfusion. Meanwhile, posttreatment with KBA significantly decreased malondialdehyde (MDA) levels, restored the superoxide dismutase (SOD) activity, and increased the protein Nrf2 and HO-1 expression in brain tissues. In primary cultured astrocytes, KBA increased the Nrf2 and HO-1 expression, which provided protection against oxygen and glucose deprivation (OGD)-induced oxidative insult. But knockdown of Nrf2 or HO-1 attenuated the protective effect of KBA.
CONCLUSIONS:
In conclusion, these findings provide evidence that the neuroprotection of KBA against oxidative stress-induced ischemic injury involves the Nrf2/HO-1 pathway.
HPLC of 11-Keto-beta-boswellic acid
