
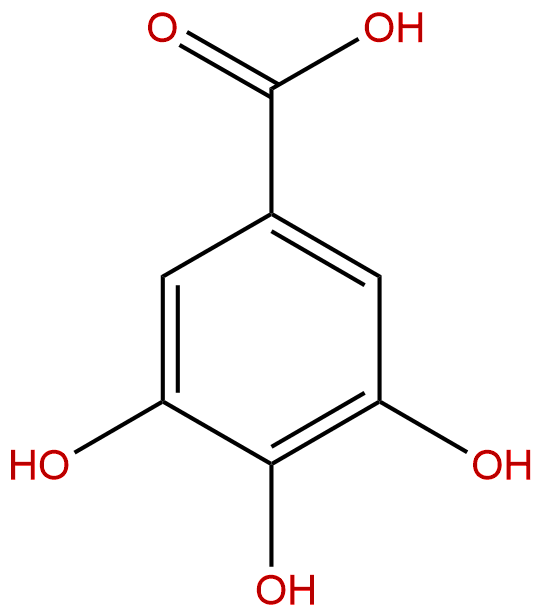
Gallic acidCAS No.:149-91-7
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0608 |
| Formula: | C7H6O5 |
| Mol Weight: | 170.12 |
Product name: Gallic acid
Synonym name: 3,4,5-Trihydroxybenzoic acid
Catalogue No.: BP0608
Cas No.: 149-91-7
Formula: C7H6O5
Mol Weight: 170.12
Botanical Source: Isol. by Scheele in 1786 from fermented galls. Occurs in many tannins. Specific flowering inhibitor in leaves of Kalanchoe bloesfeldiana
Physical Description: Powder
Type of Compound: Phenols
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams, up to kilograms
Inquire for bulk scale.
Descriptions:
Gallic acid and curcumin are natural phenolic compounds, both have cytotoxic activity in relation to their radical modulating activity, induce apoptosis by different mechanisms, and gallic acid produces higher amounts of radicals and more efficiently scavenged the superoxide anion radical.[1]
Gallic acid has antioxidant activities at a higher concentration is mainly due to the scavenging of hydrogen peroxide in this system, and the pro-oxidant mechanism is most likely due to the strong reducing power and weak metalchelating ability.[2]
Gallic acid (GA) has a strong anti-tyrosinase activity (IC50=3.59x10(-6) M), effectively suppresses murine tyrosinase action and the amount of melanin, down-regulates the RS generation and enhanced the glutathione (GSH)/oxidized glutathione (GSSG) ratio, indicates that GA exerts antimelanogenic activity coupled with antioxidant properties by suppressing RS generation and maintaining a higher glutathione (GSH)/oxidized glutathione (GSSG) ratio.[3]
Gallic acid, a histone acetyltransferase inhibitor, can efficiently block neuronal cell death by downregulating the expression of cytokines and the in vivo levels of NF-κB acetylation, suggest that GA is a possible therapeutic approach for alleviating the inflammatory progression of Alzheimer disease.[4]
Gallic acid is a potent inhibitor of brush border sucrase and other disaccharidases and thus could potentially interfere with the digestive functions of the intestine.[5]
Gallic acid has anti-tumor activity, combination of gallic acid and cisplatin increased the tumor cell apoptosis compared with the treatment with cisplatin alone, suggests that the combination of gallic acid with an anti-cancer drug, including cisplatin, may be an effective protocol for lung cancer therapy.[6]
References:
[1] Nogaki A, Satoh K, Iwasaka K, et al. Anticancer Res, 1998, 18(5A):3487-91.
[2] Yen G C, Duh P D, Tsai H L. Food Chem, 2002, 79(3):307-13.
[3] Kim Y J. Biol Pharm Bull, 2007, 30(6):1052-5.
[4] Kim M J, Seong A R, Yoo J Y, et al. Mol Nut Food Res, 2011, 55(12):1798–808.
[5] Gupta N, Gupta S, Mahmood A. Nut Res, 2007, 27(4):230-5.
[6] Kawada M, Ohno Y, Ri Y, et al. Anti-cancer Drugs, 2001, 12(10):847-52.
[7] Sawant L, Prabhakar B, Pandita N. J Anal Bioanal Techniques, 2010, 1(1):1-4.
HPLC of Gallic acid
